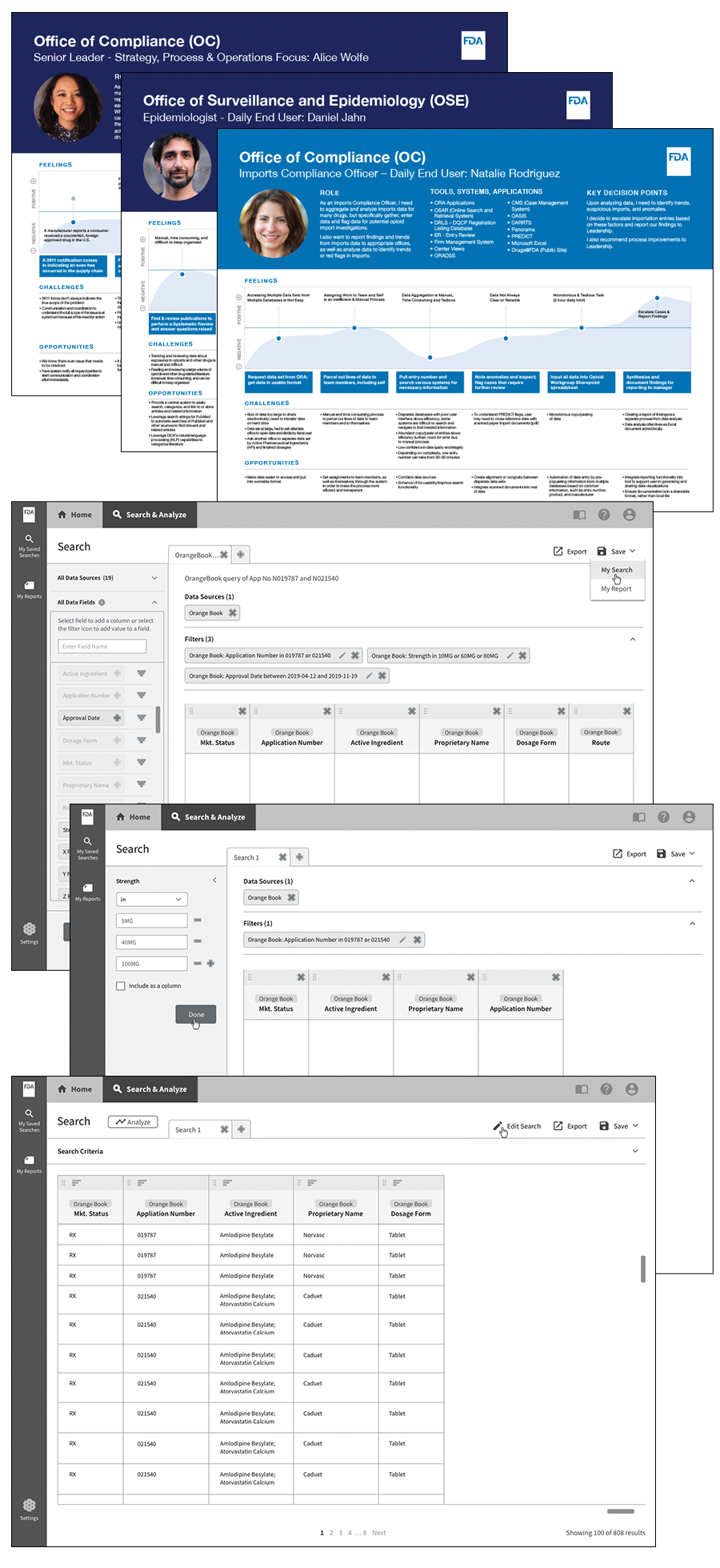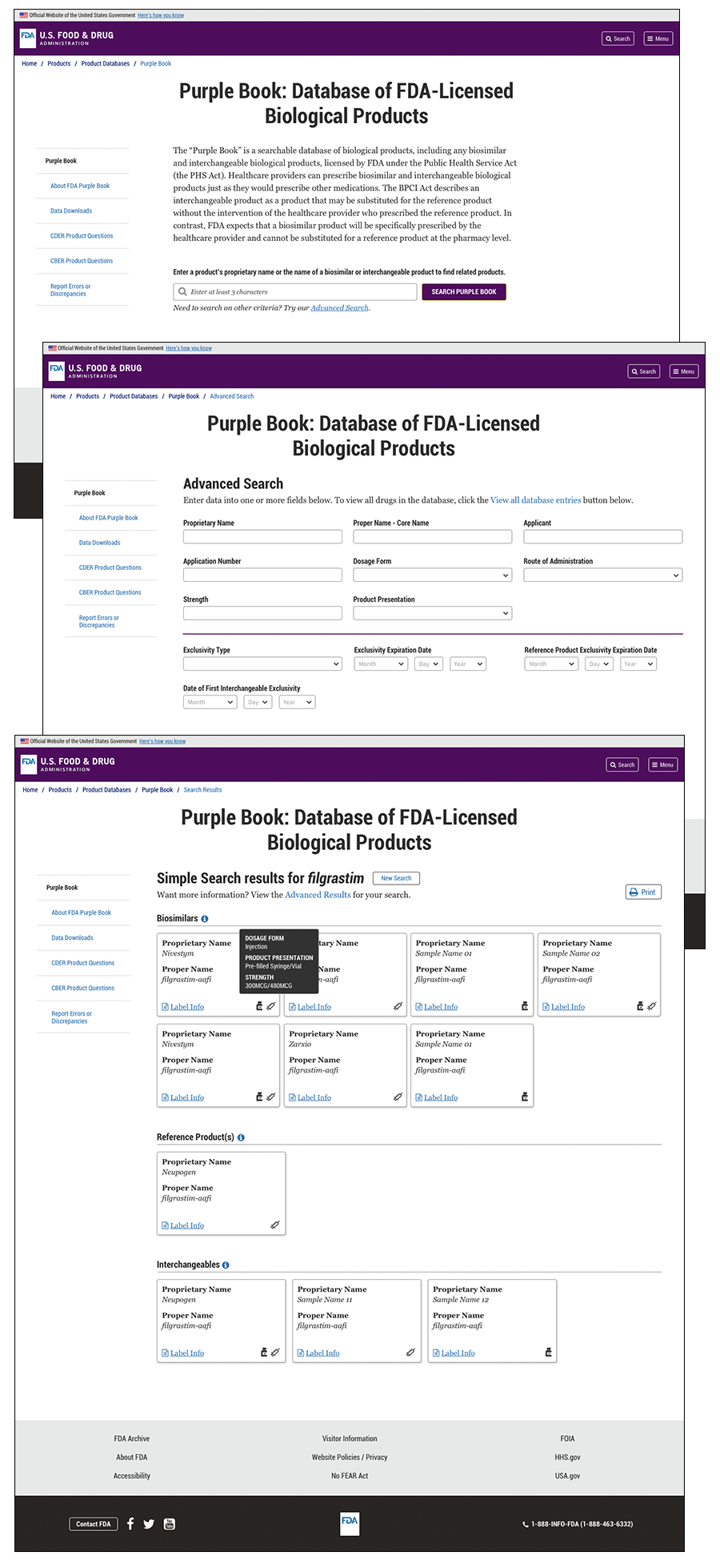
My Role: Lead Designer/Digital Transformation Specialist
Challenge
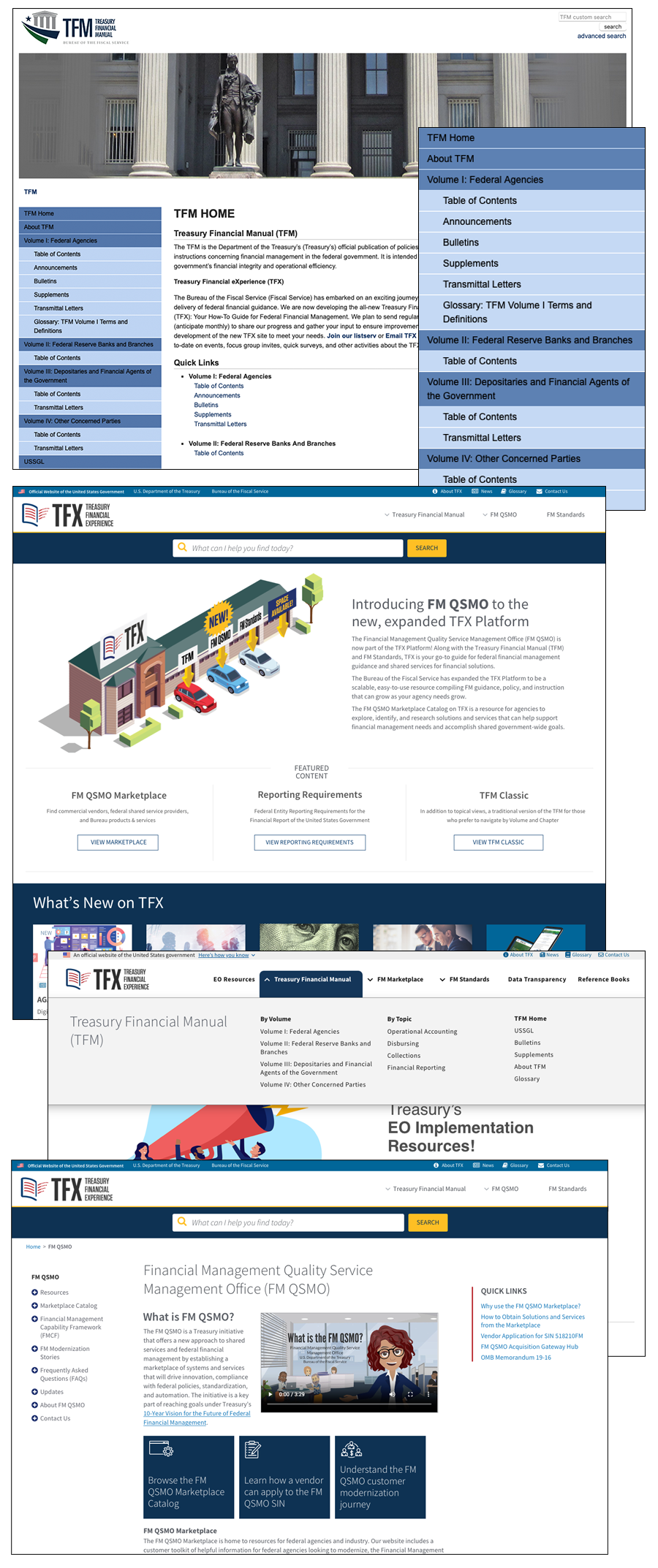
The Treasury Financial Manual (TFM) was originally a series of binders outlining how agencies conduct business with the Federal Government. When it was put online, it maintained its volume/part/chapter setup, but users wanted topic-based content, better search capabilities, and access to related resources.
Solution
Instead of just reorganizing content, we re-imagined what digital Federal Financial Management (FFM) could be and built the Treasury Financial Experience (TFX) platform: a scalable, easy-to-manage Drupal WCMS solution that could grow as we consolidated FFM resources from across Fiscal Service and Treasury. Working with users and stakeholders in focus groups and card sorts, we developed an intuitive topic-based information architecture while maintaining the legacy book structure view, all from one content repository.
Impact/Results
One-Stop Shop for FFM Policy, Guidance, and Instruction
Users no longer need to go to multiple resources for answers on how their agency conducts business with the Federal Government.
Streamlined Operations & Maintenance
Migrated six (6) additional resources into the platform, including Financial Management (FM) Standards, FM QSMO Marketplace, Data Transparency, Green Book, Gold Book, and FAST Book, reducing the Bureau-wide burden of system O&M.
20% Reduction in Content
With redundant, outdated, and trivial (ROT) content analysis of each system to be migrated, we reduced content by over 20% while streamlining existing content for easier access and comprehension.
Create Once, Publish Everywhere
Our COPE content model and tagging structure allows for views of critical related content in multiple areas, both within the TFX Drupal platform and externally using APIs, all from one central repository.
Improved Content Workflows
The publishing of financial policy and guidance requires specific roles and approval procedures under Federal law, all enabled by Drupal’s robust workflows.